By Brendan Pierson
A federal appeals court in New Orleans will hear arguments on Wednesday in a closely watched case brought by anti-abortion activists seeking to ban the abortion pill mifepristone, with potentially far-reaching impact on abortion access across the United States.
The Biden administration will urge a three-judge panel of the 5th U.S. Circuit Court of Appeals to overturn last month's unprecedented ruling by U.S. District Judge Matthew Kacsmaryk in Amarillo, Texas suspending mifepristone's FDA approval. Danco Laboratories, which sells the drug under the brand name Mifeprex, is also expected to argue before the panel.
Biden's administration is seeking to defend mifepristone in the face of mounting abortion bans and restrictions enacted by Republican-led states since the U.S. Supreme Court in June 2022 overturned the landmark 1973 Roe v. Wade decision that had legalized the procedure nationwide.
Anti-abortion groups and doctors, led by the recently formed Alliance for Hippocratic Medicine, will be defending Kacsmaryk's order. They claimed in their lawsuit last year that mifepristone is unsafe and that the U.S. Food and Drug Administration's approval, almost 23 years ago, was illegal.
The administration is expected to argue that the plaintiffs have no standing to bring the case, because they are not harmed by mifepristone's approval, and that the drug's safety is supported by decades of data and real world use.
By filing their case in Amarillo, the plaintiffs assured it would go before Kacsmaryk, a conservative and former Christian activist, and that any appeal would go the conservative 5th Circuit. Twelve of the circuit's 16 active judges were appointed by Republican presidents.
All three judges on Wednesday's panel are staunchly conservative, with a history of opposing abortion rights.
Mifepristone remains available for now, following an emergency order from the U.S. Supreme Court putting Kacsmaryk's order on hold during the appeal.
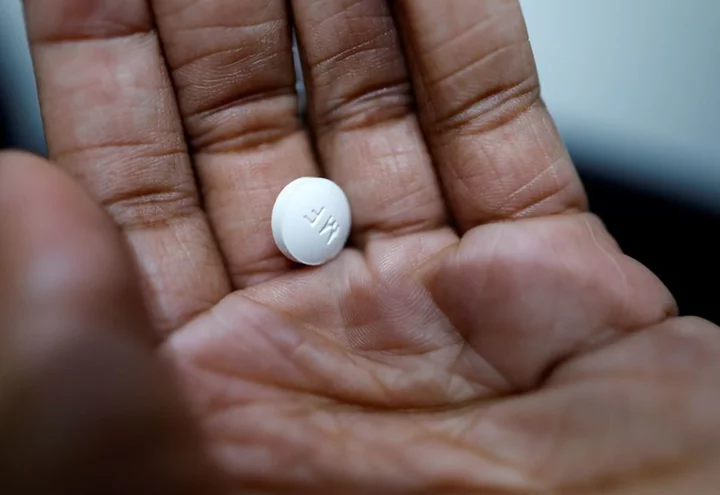
Mifepristone is part of a two-drug regimen with misoprostol used for medication abortions, which account for more than half of U.S. abortions. It is approved for use in the first 10 weeks of pregnancy.
Numerous medical studies have concluded that the drug is safe and effective.
Major medical associations, including the American College of Obstetricians and Gynecologists (ACOG) and the American Medical Association (AMA), have said in court filings that pulling mifepristone off the market would harm patients by forcing them to undergo more invasive surgical abortions.
Drug industry groups have also said it would disrupt drug development by making every FDA approval subject to second-guessing by courts.
Those groups, including Pharmaceutical Research and Manufacturers of America, are among many organizations and people that have submitted friend-of-the-court briefs in the case.
The AMA, ACOG and Democratic lawmakers have also weighed in to support the administration, while anti-abortion groups and Republicans have backed the plaintiffs.
Whichever way the 5th Circuit panel rules, the decision is sure to be appealed, first to the full court and then to the U.S. Supreme Court.
(Reporting By Brendan Pierson in New York, Editing by Alexia Garamfalvi and Bill Berkrot)