TOKYO--(BUSINESS WIRE)--Aug 4, 2023--
Duchenne Muscular Dystrophy (DMD) patients showed signs of disease progress slowing down, after oral consumption of Neu REFIX ß-glucan for 45 days along with routine medications. Clinical improvements published in IBRO Neuroscience reports, might be the world's first such report, wherein, a safe food supplement, mitigated biomarkers of relevance to muscle dysfunction in DMD without side-effects. Increased plasma dystrophin by 32%, indicating improved blood circulation along with muscle strength improvement, is a breakthrough, unraveling the multipronged potential of Neu-REFIX, which also beneficially reconstituted gut microbiome in DMD, gives hope of improving the quality of life and prolonging the life span of DMD patients, opined Dr. Raghavan, the lead author.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230803923960/en/
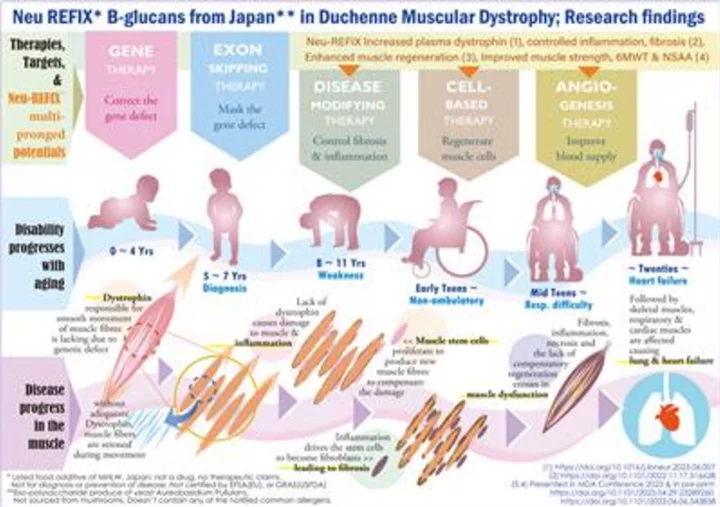
Duchenne Muscular Dystrophy (DMD); Progress of disease & gradual disability with aging, current therapies & Neu-REFIX Beta glucans' multipronged potentials, illustrated. A rare genetic disease with approximately 5000 patients in Japan, 3000 in GCC, fewer than 50000 in USA. Lack of dystrophin in DMD causes muscle dysfunction and makes the patient wheelchair bound in early teens. Lung function deterioration followed by myocardial fibrosis and heart failure causing early death in 20s ~ 30s. Gene therapy targets gene defect correction, exon skipping therapies mask the defect of specific exons. Safety proven Neu-REFIX Beta glucans from Japan, a food additive with multipronged potentials: (i) controlled muscle damage, (ii) enhanced muscle regeneration (iii) improved blood supply marker: plasma dystrophin & (iv) improved 6MWT & NSAA, yielding hope of delaying the disease progress, worth further validation; is not a drug or remedy; Not GRAS or EFSA certified. Approval status varies country wise. (Graphic: Business Wire)
Neu-REFIX is an allergen-free ß-glucan, unique for its source, as an exo-polysaccharide from Aureobasidium Pullulans, produced in GMP certified facility in Japan, possessing purity, functionality, water solubility and dosage standardization like a drug, preservable at room temperature, and orally consumable as such or with food. Immune modulatory effects in pre-clinical studies, in healthy Japanese volunteers and Covid-19 patients, showed its potentials as a disease modifying adjuvant, worth undertaking clinical trial in DMD, to yield beneficial effects by controlling inflammation and fibrosis.
Neu-REFIX reduced fibrosis, inflammation, enhanced muscle regeneration, in MDX mice and in a six-month-long clinical study in young boys, improved NSAA, 6MWT and MRC, and these disease modifying potentials were presented in MDA Conference 2023. Neu-REFIX, can be consumed at any stage of DMD, with standard of care medications. Therefore, it’s worth evaluating as a drug-adjuvant because, as reported, Neu-REFIX as a single agent, has helped accomplish, what the current disease modifying treatments, cell-based therapies, and angiogenesis approaches are together, trying to achieve, said Prof. Naoki Yamamoto, a co-researcher.
GNCorp, which spearheaded this accomplishment, has signed a MoU with MAP Healthcare, a subsidiary of SHBK International Holding Group, UAE and intends collaborating with like-minded organizations to validate Neu-REFIX in larger studies. Clinical research in Limb-Girdle Muscle Dystrophy (LGMD),multiple sclerosisand psoriasis are underway, to evaluate Neu-REFIX’s efficacy as a universal immune modulator.
*B-1,3-1,6 glucan is alisted food additive in MHLW, Japan; Not a drug or remedy to any illness. Research findings should not be construed as medical advice. Not GRAS, EFSA certified.
View source version on businesswire.com:https://www.businesswire.com/news/home/20230803923960/en/
CONTACT: Samuel JK Abraham
info@gncorporation.com
KEYWORD: TEXAS UNITED STATES JAPAN NORTH AMERICA ASIA PACIFIC
INDUSTRY KEYWORD: SCIENCE RESEARCH HEALTH GENETICS FOOD/BEVERAGE FITNESS & NUTRITION CLINICAL TRIALS RETAIL
SOURCE: GN Corporation Co Ltd
Copyright Business Wire 2023.
PUB: 08/04/2023 05:02 AM/DISC: 08/04/2023 05:01 AM
http://www.businesswire.com/news/home/20230803923960/en