DALLAS--(BUSINESS WIRE)--Jun 8, 2023--
Lantern Pharma Inc. (NASDAQ: LTRN), a clinical-stage biopharmaceutical company using its proprietary RADR ® artificial intelligence ("AI") and machine learning (“ML”) platform to transform the cost, pace, and timeline of oncology drug discovery and development, today announced it will be leveraging its AI platform, RADR ®, in a research collaboration with Bielefeld University (Bielefeld, Germany) to develop antibody-drug conjugates (ADCs) with high therapeutic and antitumor potential. The collaboration will leverage insights from Lantern’s recently developed RADR ® AI ADC module in combination with research from Professor Norbert Sewald, Ph.D., the principal investigator for Bielefeld and leader of Magicbullet::reloaded, a European consortium focused on developing novel drug delivery mechanisms, including ADCs. Outcomes from the collaboration are expected to pave the way for next-generation ADCs and other drug conjugates that are designed using AI and that can be developed with potentially higher efficacy, at a faster pace, and with significantly reduced costs.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230608005298/en/
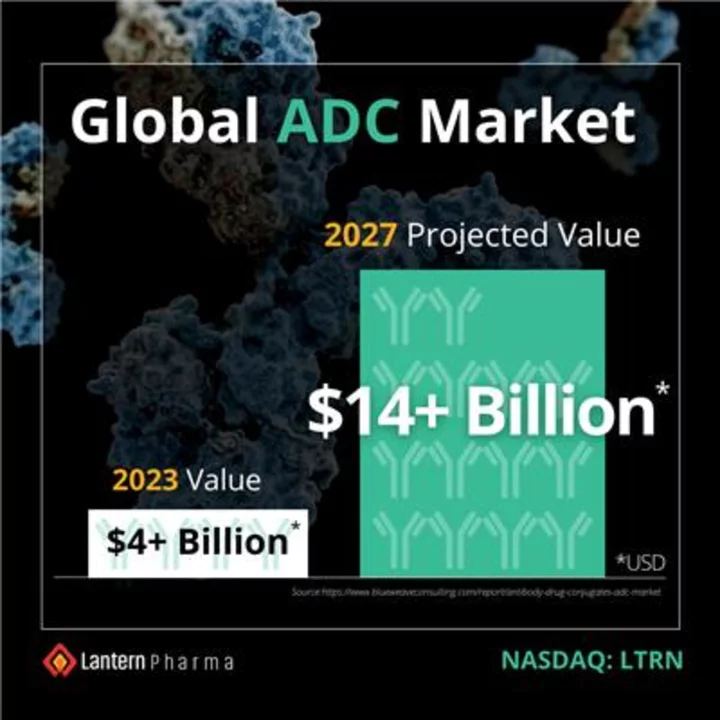
The global ADC market is currently over $4.0 billion and is projected to reach $14.0 billion by 2027 (Graphic: Business Wire)
The initial aim of the collaboration will be to synthesize and evaluate novel ADCs linked to cryptophycins, which are promising antitumor molecules, in part because of their potency at ultra-low, picomolar, concentrations. The cryptophycin-ADCs will be tested across multiple cancer cell lines and initial results are expected during 2023.
”We have ample experience in structure-activity relationships of cryptophycins as well in the synthesis of ADCs and small molecule-drug conjugates (SMDCs). Teaming up with top researchers from European academia and industry in the consortium of Magicbullet::reloaded further reinforced this capacity. We now look forward to the collaboration with Lantern,” said Dr. Sewald.
“Cryptophycins are an exciting family of highly potent heterocyclic peptides from Cyanobacteria that have demonstrated antitumor potency and can inhibit tumor growth by strongly interfering with microtubule stability and assembly,” stated Kishor Bhatia, Ph.D., Lantern’s Chief Scientific Officer. “Dr. Sewald and his group are experts in the synthesis of cryptophycin derivatives and have established extensive groundwork to support the targeted ADC delivery of cryptophycins. By leveraging our RADR ® platform’s AI ADC development module and partnering with Dr. Sewald, we expect to be able to select and advance cryptophycin-ADCs towards the clinic with better targeting and therapeutic efficacy for patients with advanced cancers with limited therapeutic options,” continued Dr. Bhatia.
After the initial aims of the collaboration are completed, Lantern plans to leverage its AI ADC development module, which is fully integrated into RADR ®, to launch multiple ADCs that can leverage cryptophycins or other promising payloads. Lantern also expects to use the AI ADC development module with other collaborators, both academic and commercial, to develop promising ADC candidates for launch into targeted clinical trials.
This AI-guided strategy has the potential to de-risk the ADC drug development process, while simultaneously enhancing the creation of effective and targeted ADCs. The rapidly growing global ADC market is currently valued at over $4.0 billion and is projected to reach $14.0 billion by 2027. There are currently 12 ADCs that have been approved by the US Food and Drug Administration (FDA) for the treatment of cancer and approximately 37 ADCs in current late-stage oncology trials.
Under the terms of the collaboration, Dr. Sewald and his group will synthesize, optimize, and provide initial testing of the cryptophycin-ADCs. Lantern is also receiving an exclusive and worldwide option to license intellectual property (IP) from Bielefeld University related to the collaboration and IP generated from the collaboration.
About Norbert Sewald, Ph.D.:
Dr. Sewald is a Professor of Organic and Bioorganic Chemistry at Bielefeld University in Bielefeld, Germany. His research group’s focus includes the development of antibody-drug and peptide-drug conjugates, the isolation and total synthesis of natural products, the chemical modification of bioactive peptides, and the biocatalytic halogenation of amino acids, peptides, and proteins. Dr. Sewald is also a lead investigator of a European consortium called Magicbullet::reloaded, which is focused on investigating the field of ADCs, peptide-drug conjugates, and small molecule-drug conjugates to stimulate tumor immune responses and overcome resistance to immunotherapy.
Dr. Sewald received his Ph.D. in Organic Chemistry from the Technical University of Munich, Munich, Germany, and completed his Postdoctoral research training in the lab of Prof. Sir J.E. Baldwin, Dyson Perrins Laboratory at the University of Oxford, Oxford, UK. He has served as a full Professor at Bielefeld University since 1999.
About Lantern Pharma:
Lantern Pharma is an AI company transforming the cost, pace, and timeline of oncology drug discovery and development. Our proprietary AI and machine learning (ML) platform, RADR ®, leverages over 25 billion oncology-focused data points and a library of 200+ advanced ML algorithms to help solve billion-dollar, real-world problems in oncology drug development. By harnessing the power of AI and with input from world-class scientific advisors and collaborators, we have accelerated the development of our growing pipeline of therapies including eleven cancer indications and an antibody-drug conjugate (ADC) program. On average, our newly developed drug programs have been advanced from initial AI insights to first-in-human clinical trials in 2-3 years and at approximately $1.0-2.0 million per program.
Our lead development programs include two Phase 2 clinical programs and multiple upcoming Phase 1 clinical trials anticipated for 2023. We have also established a wholly-owned subsidiary, Starlight Therapeutics Inc., to focus exclusively on the clinical execution of our promising therapies for CNS and brain cancers, many of which have no effective treatment options. Our AI-driven pipeline of innovative product candidates is estimated to have a combined annual market potential of over $15 billion USD and have the potential to provide life-changing therapies to hundreds of thousands of cancer patients across the world.
Please find more information at:
Website: www.lanternpharma.com
LinkedIn: https://www.linkedin.com/company/lanternpharma/
Twitter: @lanternpharma
Lantern Pharma Newsletter – The Spark: Sign-up here
Forward-looking Statements:
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements include, among other things, statements relating to: future events or our future financial performance; the potential advantages of our RADR ® platform in identifying drug candidates and patient populations that are likely to respond to a drug candidate; our strategic plans to advance the development of our drug candidates and antibody drug conjugate (ADC) development program; estimates regarding the development timing for our drug candidates and ADC development program; expectations and estimates regarding clinical trial timing and patient enrollment; our research and development efforts of our drug discovery and ADC programs and the utilization of our RADR ® platform to streamline the drug development process; our intention to leverage artificial intelligence, machine learning and genomic data to streamline and transform the pace, risk and cost of oncology drug discovery and development and to identify patient populations that would likely respond to a drug or ADC candidate; estimates regarding patient populations, potential markets and potential market sizes; sales estimates for our drug and ADC candidates and our plans to discover and develop drug and ADC candidates and to maximize their commercial potential by advancing such candidates ourselves or in collaboration with others. Any statements that are not statements of historical fact (including, without limitation, statements that use words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," "model," "objective," "aim," "upcoming," "should," "will," "would," or the negative of these words or other similar expressions) should be considered forward-looking statements. There are a number of important factors that could cause our actual results to differ materially from those indicated by the forward-looking statements, such as (i) the impact of the COVID-19 pandemic, (ii) the risk that our research and the research of our collaborators may not be successful, (iii) the risk that none of our product candidates has received FDA marketing approval, and we may not be able to successfully initiate, conduct, or conclude clinical testing for or obtain marketing approval for our product candidates, (iv) the risk that no drug product based on our proprietary RADR ® AI platform has received FDA marketing approval or otherwise been incorporated into a commercial product, and (v) those other factors set forth in the Risk Factors section in our Annual Report on Form 10-K for the year ended December 31, 2022, filed with the Securities and Exchange Commission on March 20, 2023. You may access our Annual Report on Form 10-K for the year ended December 31, 2022 under the investor SEC filings tab of our website at www.lanternpharma.com or on the SEC's website at www.sec.gov. Given these risks and uncertainties, we can give no assurances that our forward-looking statements will prove to be accurate, or that any other results or events projected or contemplated by our forward-looking statements will in fact occur, and we caution investors not to place undue reliance on these statements. All forward-looking statements in this press release represent our judgment as of the date hereof, and, except as otherwise required by law, we disclaim any obligation to update any forward-looking statements to conform the statement to actual results or changes in our expectations.
View source version on businesswire.com:https://www.businesswire.com/news/home/20230608005298/en/
CONTACT: Nicole Leber
Investor Relations Associate
ir@lanternpharma.com
KEYWORD: UNITED STATES NORTH AMERICA TEXAS
INDUSTRY KEYWORD: TECHNOLOGY RESEARCH BIOTECHNOLOGY HEALTH PHARMACEUTICAL GENERAL HEALTH SCIENCE ARTIFICIAL INTELLIGENCE ONCOLOGY
SOURCE: Lantern Pharma
Copyright Business Wire 2023.
PUB: 06/08/2023 09:00 AM/DISC: 06/08/2023 09:01 AM
http://www.businesswire.com/news/home/20230608005298/en