Sangamo Therapeutics Inc.’s cost cuts didn’t hurt just the roughly 162 US workers who lost their jobs this month. It’s also a blow to Jerry Walter, who’s lost five family members and suffered kidney, lung, hearing and heart damage from a rare disease.
Citing the “challenging economic environment” that’s sending shock waves through the biotech industry, Sangamo paused research on an experimental gene therapy — possibly a cure — for Fabry disease. Walter, a 69-year-old retired US Army colonel who’s already had a heart transplant due to the inherited enzyme deficiency, said it’s disappointing to see work stop on yet another potential new medicine.
“The promise of a better treatment keeps getting pushed down the road,” he said.
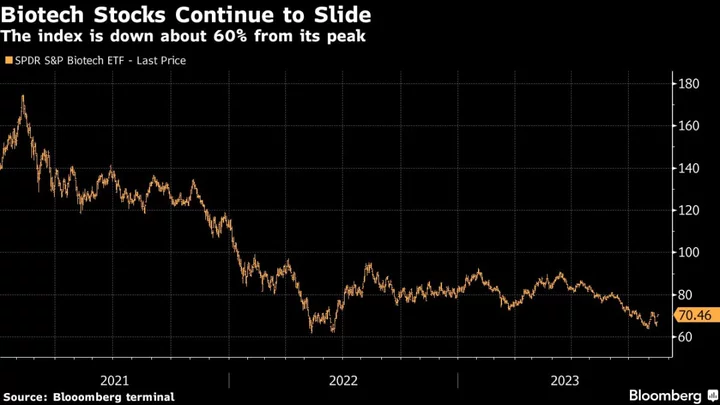
Sangamo will finish a current Fabry trial as planned and is seeking funding or a partner for help with moving its studies to the final stage, a spokesperson said. Yet it’s been another bleak year for biotech companies as a closely watched index of stocks, often called the XBI, just passed 1,000 days from its early 2021 peak. The number of companies exploring strategic options — typically a sign of desperation — just hit a record high, according to analysts from Stifel.
“Nobody knows where the bottom of this is,” said Sam Fazeli, an analyst at Bloomberg Intelligence.
Money for research is getting harder to find: New flows into biotech venture funds are estimated to fall to $23 billion this year from about $31 billion in 2021, the Stifel analysts said. Rising interest rates, threats to prices of new drugs and a post-pandemic letdown have forced biotechs to pull back on research and development.
Last year, global pharma R&D spending fell 2.2% to $244 billion, the first drop in a decade, according to Evaluate Pharma, and slowed growth is expected in the coming years. It was just the fourth such decline in almost four decades, according to the PhRMA industry group.
The sector has seen “serious slimming down of pipelines at the small end,” Evaluate said in its August report.
Crucial Work
Big drugmakers have increasingly moved into areas that used to be biotech’s domain — like cancer and rare diseases — seeking profits from drugs that aren’t easily replaced by competitors. French drugmaker Sanofi is boosting R&D spending, while Eli Lilly & Co., maker of blockbuster weight-loss drugs, increased this year’s R&D budget by $600 million.
But the industry giants can’t replace the crucial work done by smaller biotech companies, said Derek Lowe, a longtime drug researcher who writes the industry blog “In the Pipeline.” In many cases, biotech companies handle risky early-stage research that big drugmakers may invest in if it shows promise.
Biotech companies often take on challenges that are a bit “out there,” Lowe said. Big manufacturers “let the little companies take a crack at it and see how it goes.”
Gone is the pandemic enthusiasm that drove Covid shot-maker Moderna Inc.’s 12-month trailing price as high as 188 times diluted earnings per share from continuing operations in June 2021. Now the company is expected to report losses on an adjusted basis over the next year, and more than half the analysts covering it don’t recommend buying shares.
An industry slowdown was inevitable, Fazeli said. Firms are shifting their strategies in response. Unable to find investors who can provide more funds, small biotechs are skipping the long shots to focus on sure bets that are more likely to generate income soon or attract a deal with a bigger drug company, said John LaMattina, a former head of R&D at Pfizer Inc.
That means less creativity in the sector that gave rise to Covid shots, life-saving gene therapies and other treatments for once-fatal diseases. Those include Merck & Co.’s Keytruda, which first took shape about two decades ago at biotech company Organon & Co. Today the drug has treated more than 1 million patients with more than 20 different cancers, generating some $21 billion in sales last year.
Medicine Paucity
“The concern is that in three or four years from now there will be a paucity of innovative medicines,” said Jeff Jonas, former chief executive officer of the biotech company Sage Therapeutics Inc. “There is potential for the early ecosystem to suffer.”
The impact is hitting companies like Charles River Laboratories International Inc. that does work including early safety research on rats that precedes human testing. The firm has been involved in studying more than 80% of all US-approved drugs in the past three years.
Biotech companies that once investigated four or five products at a time now just have enough funding for two, said Jim Foster, Charles River’s chief executive officer. The result: organic revenue is expected to grow about 6% this year, roughly half its 2022 level.
“People are reprioritizing, being more judicious,” Foster said in an interview. “We are a canary in the coal mine.”
Lab space vacancies in the Boston biotech industry hub have hit a 10-year high, according to the real estate firm Colliers. For the first time, there’s about 5 million square feet of space on the market, up from 300,000 two years ago.
IRA Fears
Biotech’s woes could be a problem for big drugmakers, said Lowe, the pharma blogger: a smaller crop of candidates for big drug companies to buy later on. Those drugmakers need new products to make up for older ones that will be facing competition when their exclusivity runs out.
Aging drugs are also eligible for Medicare price negotiations under the Inflation Reduction Act, President Joe Biden’s signature legislation. Drugmakers have said that the law effectively shortens their products’ most profitable period, and that some early development programs will have to be sacrificed.
All that is bad news for Walter, the former colonel who founded the National Fabry Disease Foundation. Twice a month, the Hillsborough, North Carolina, resident sits for three hours getting enzyme replacement through an IV.
Before running short of money, Sangamo was pursuing a one-time treatment aimed at fixing the gene flaw responsible for the disease, meaning Fabry patients wouldn’t need those twice-monthly infusions. Those who got it in a trial “continue to report improvements in their quality of life,” the company said.
The treatment could have been “revolutionary,” had work on it continued, Walter said, adding he hopes that Sangamo can resolve its funding issues.
(Corrects frequency of patient’s infusions in final section)